 |
| Different Types of Simple Carboxylic Acids |
Carboxylic
Acids
 |
| Carboxylic-acid-group-3D |
Naming Carboxylic Acids:
 |
| Carboxylic Acids Slide 1 |
The parent chain must include the carboxyl carbon, which is given
position number 1. The name of the alkane attached is changed by replacing the -e
with -oic acid. For example:
|
HCOOH
methanoic acid
|
CH3COOH ethanoic acid (acetic acid)
|
CH3CH2CH2COOH butanoic
acid
|
Because carboxylic acids have both a lone oxygen and an OH group,
they are strongly hydrogen-bonded to each other, therefore having high boiling
points.
 |
| Carboxylic Acids Slide2 |
 |
| Ethyl Ethanoate |
The carboxyl group is weakly acidic and all carboxylic acids
neutralize OH-.
RCOOH + NaOH ==> RCOONa + H2O
Esters of Carboxylic Acids
Carboxylic acids are used to synthesize two important derivatives,
esters and amides. In esters, the OH of the carboxyl group is
replaced by OR.
To name esters, first have the name of the akyl group attached to
the O, followed by the name of the parent carboxylic acid after its name is
altered by changing -ic acid to -ate. For exmample:
CH3COO(CH2)7CH3
octyl ethanoate
 |
| Naming of Esters |
In an mixture with carboxylic alcohol, an equilibrium reaction
forms ester and water in the presence of heat and a strong acid catalyst.
RCOOH + HOR' <==> RCOOR' + H2O
For example:
CH3CH2CH2COOH
+ HOCH2CH3 <==> CH3CH2CH2COOCH2CH3
+ H2O
butanoic acid + ethanol <==>
ethyl butanoate + water
Hydrolysis and Saponification of
Esters
 |
| Hydrolysis of Ester |
An ester is hydrolyzed to its parent acid and alcohol when it is
heated together with a stoichiometric excess of water (with acid
catalyst)--this is the reverse of the reaction above. Saponification, is
the reaction of an ester with a strong base, like sodium hydroxide. For
example:
CH3COOCH2CH3
+ NaOH ==> CH3COO- + Na+ + HOCH2CH3
ethyl ethanoate + sodium hydroxide
==> ethanoate ion + sodium ion + ethanol
\________________________/
|
sodium ethanoate salt
The mixtures of salt of long-chain carboxylic acids are the
ingredients of ordinary soap.
 |
| Functional Groups |
Amides of Carboxylic Acids
In amides, the OH of the carboxyl group is replaced by nitrogen
holding any combination of H atoms or hydrocarbon groups.
The names of simple amides are devised by writing the name of the
parent carboxylic acid and replacing the ending, -oic acid, with -amide
For example:
CH3CONH2
ethanamide (acetamide)
The preparation of simple amides are similar to those of esters,
but they are in an excess of ammonia. For example:
RCOOH + NH3 ==> RCONH2
+ H2O
carboxylic acid + ammonia ==>
simple amide + water
When amides are in water, they are changed back to the carboxylic
acid and ammonia.
Unlike amines, the amides are nonbasic even though they have the
NH2 group in simple amides. This is because of the O atom in the
carbonyl group, which is very electronegative. This tightens the electrons on N
so that it is unable to accept a proton.
Methods of Preparation:
 |
| Methods of Preparation |
1) Oxidation of primary alcohols:
KMnO4
R---CH2OH
------------> R---COOH
2) Oxidation of alkylbenzenes:
KMnO4
C6H5---R
-----------> C6H5---COOH
K2Cr2O7
3) Carbonation of Grignard reagent:
H(+)
R---MgX + O
= C = O --------> R---COO(-)MgX(+) ------> R---COOH + Mg(2+) + X(-)
4) Hydrolysis of nitriles:
R---C = N + 2H2O
------------> R---COOH + NH3
Nitriles may be prepared by adding NaCN to the corresponding
halides. for example:
H2O, H(+)
CH3CH2CH2Br + CN(-)
--------------------> CH3CH2CH2CN
--------------> CH3CH2CH2COOH
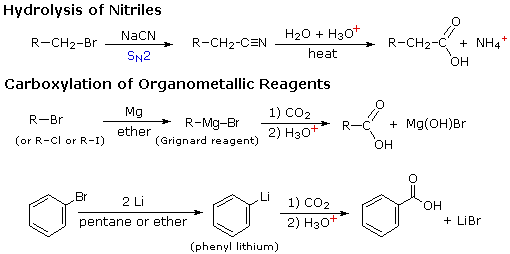 |
| Hydrolysis of nitriles: |
The above substitution of X with CN is applicable only when the
halide is a primary halide.Tertiary halides yield alkene and for secondery
halide, the yield of substitution product is poor.
Physical Properties:
The molecules of carboxylic acids are polar and exibit hydrogen
bonding.The bonding point of a carboxylic acid is higher than that of an
alcohol of comparable molar mass.This is due to the fact that the carboxylic
acids exit as dimer.
Chemical Reactions:
1) Acidity: Carboxylic acids are weak acids and their
carboxylate anions are strong conjugate bases.The aqueous solutions of
carboxylate salts are slightly alkaline due to the hydrolysis of carboxylate
anion. Compared to other species, the orders of acidity and basicity of
corresponding conjugate bases are as follows.
_
Acidity : RCOOH > HOH
> ROH >HC = CH > NH3 > RH
_
Basicity:RCOO(-) <
HO(-) < RO(-) < HC = C(-) < NH2 (-) <
R(-)
Carboxylic acids react with metals to liberate hydrogen and are
soluble in both NaOH and NaHCO3
solutions. For example:
2CH3COOH + 2Na
-------> 2CH3COO(-)Na(+) + H2
CH3COOH +
NaOH-------->CH3COO(-)Na(+) + H2O
CH3COOH +
NaHCO3 ----------> CH3COO(-)Na(+)
+ H2O + CO2
2) Conversion to acid chloride:This may
be carried out by using thionyl chloride(SOCl2),
Phosphorus trichloride(PCl3)and
phosphorus pentachloride(PCl5).
Thionyl chloride is more convenient as the side products are only gaseous and
thus the acid chloride can be easily separated; any excess of SOCl2 can be
easily removed as its boiling point is low (79`C).
RCOOH +
SOCl2 ----------------> RCOCl + SO2 + HCl
reflux
RCOOH + PCl5
----------------> RCOCl + POCl3 + HCl
Heat
3) Conversion into esters:
SOCl2 R`OH
RCOOH
------------> RCOCl-----------> RCOOR
acid acid
chloride ester
A direct reaction between acid and alcohol is a reversible one.
Ester can be obtained either by using one of the reactants in excess or b y
removing one of the products.
H(+)
RCOOH +
R`OH <------> RCOOR` + H2O
H(+) acts as a catalyst.The
presence of bulky groups near the site of reaction, whether in the alcohol or
in the acid, slows down esterification as well as its hydrolysis.The relative
order of esterification is.
CH3OH > 1`
> 2` > 3`
HCOOH > CH3COOH >
RCH2COOH > R2CHCOOH > R3CCOOH
The accepted mechanism is as follows.
Mineral acid speeds up both processes by protonating carbonyl
oxygen and thus rendering carbonyl carbon more susceptible to nucleophilic
attack.In esterification, the nucleophile is R`OH and leaving group is water
and inthe hydrolysis, the roles are reversed.
4) Reduction of acids to alcohol: Lithium
aluminium hydride, LiAlH4, is used to convert acids into
alcohols. The initial product is an alkoxide which on hydrolysis gives an
alcohol.
4R---COOH +
3LiAlH4 -----------> 4H2 + 2LiAlO3 + (RCH2O)4AlLi
(RCH2O)4AlLi--------------->4RCH2OH
Alternatively, an ester may be converted into alcohol by the use
of sodium metal and alcohol or LiAlH4 for
example:
CH3(CH2)14COOC2H5
----------------> CH3(CH2)14CH2OH + C2H5OH
5) Halogenation of aliphatic acids(Hell-Volhard-Zelinsky reaction): In the
pesence of phosphorus, chlorine or bromine replaces a hydrogen of an acid by halogen atom for example
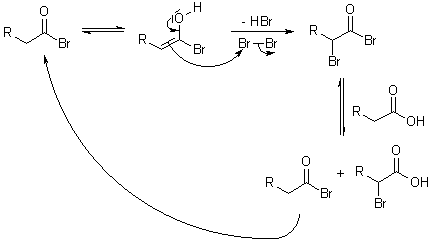 |
| (Hell-Volhard-Zelinsky reaction) |
6) Decarboxylation: Heating
of sodium salt of of carboxylic acid with soda lime (NaOH + CaO) produces
alkane
NaOH
RCOONa
----------------> RH + Na2CO3
CaO
Heating of calcium salt of carboxylic acid producs the compound
containing CO group.
RCO2(Ca/2) +
R`CO2(Ca/2) -------------> RCOR` + Ca2CO3
If R is H, then aldehyde
R`CHO is produced.
 |
| Chemical Reactions of Carboxylic Acids |




























 Online Movies
Online Movies
No comments:
Post a Comment