 |
| Animated Aldehydes and Ketones |
Organic chemistry :
is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes using spectroscopy and other physical and chemical methods to determine the chemical composition and constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes both their preparation—by synthesis or by other means—as well as their subsequent reactivities, both in the laboratory and via theoretical (in silico) study.
The objects of study in organic chemistry include hydrocarbons, compounds containing only carbon and hydrogen, as well as compositions based on carbon but containing other elements. Organic chemistry overlaps with many areas including medicinal chemistry, biochemistry, organometallic chemistry, and polymer chemistry, as well as many aspects of materials science.
Organic compounds form the basis of all earthly life. They are structurally diverse. The range of application of organic compounds is enormous. They either form the basis of, or are important constituents of, many products including plastics, drugs, petrochemicals, food, explosive material, and paints.
 |
| Aldehydes And Ketones |
Naming Aldehydes:
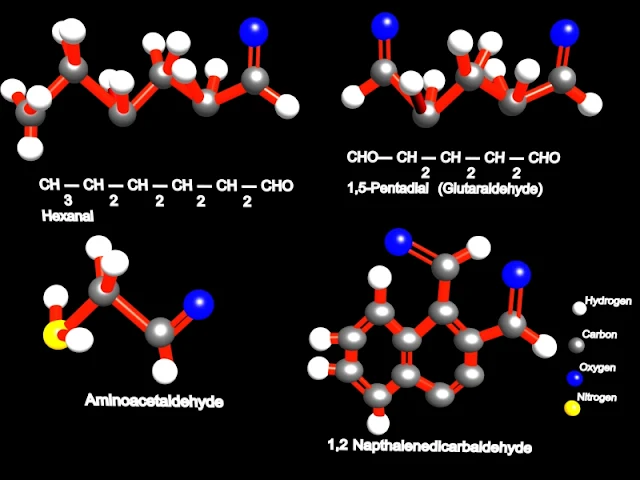
The parent chain is the longest chain that includes the aldehyde group. It's name is made by replacing the ending -e of parent alkane name with -al. The numbering of the chain always starts with the carbon of the aldehyde group being 1. For example: (parent chain will be bold for visual clarity)
H | C=O | H methanal CH3 | C=O | H ethanal CH3CH2 | C=O | H propanal CH3 | CH3CH | C=O | H 2-methylpropanal
 |
| 3D Pictures of Aldehydes |
Naming Ketones
In naming ketones, the parent chain includes the carbonyl group and is numbered so that the carbonyl location is the lowest number. The number of the location must be part of the name whenever there would be an uncertainty. For example: (parent chain will be bold for visual clarity)
CH3 | C=O | CH3 propanone (acetone) CH3CH2 | C=O | CH2CH2CH3 3-hexanone CH3 | CH3CH | C=O | CH2CH2CH3 2-methyl-3-hexanone
 |
| Methyl Ethyl Ketone |
 |
| Methyl-Vinyl-Ketone |
1) Hydrogenation of Aldehydes and Ketones
Just like the double bond in alkenes, aldehydes and ketones also add hydrogen by breaking the double bond under roughly the same conditions--metal catalyst, heat, and pressure. For example:
CH3 CH3 | | C=O + H-H ==> H-C-O-H or CH3CH2OH | | H H ethanal + hydrogen ==> ethanol CH3 CH3 OH | | | C=O + H-H ==> H-C-O-H or CH3CHCH3 | | CH3 CH3 propanone + hydrogen ==> 2-propanol
 |
| Preparation of Aldehydes |
 |
| Preparation of Ketones |
2) Oxidation of Aldehydes
Aldehydes are the most easily oxidized of all organic compounds, though ketones resist oxidation strongly. They can also be slowly oxidized by oxygen in the air. In this example, potassium dichromate, together with hydrogen sulfate, easily oxidizes ethanal to ethanoic acid:
3CH3CHO + K2Cr2O7 + 4H2SO4 ==> 3CH3COOH + Cr2(SO4)3 + K2SO4 + 4H2O
ethanal ethanoic
acid
3)Action of Grignard reagents:
H H
| |
RH---C = O + R`MgX ------------> R---C---OMgX -------------> R---C---OH
| |
R` R`
2`alcohol
4) Reactions with sodium hydrogen sulphite:
| |
C = O + NaHSO3 <---------> ---C---OH
| |
SO3Na
bisulphite
5) Addition of HCN(or sodium cyanide and mineral acid):
| | |
C = O + :CN(-) --------> ---C---O(-) -------> ---C---OH
| | |
CN CN
cyanohydrin
CN COOH
| |
CH3CH2CCH3 ------> CH3CH2CCH3 ------> CH3CH2CCH3
|| | |
O OH OH
COOH
|
CH3CH = C---CH3
2-methyl -2-butenoic acid
6) Addition of derivatives of ammonia: The derivatives of ammonia which add on the carbonyl group are hydroxylamine, hydrazine, phenylhydrazine and semicarbazide. Their stable forms are available in the form of salts;hydroxylamine hydrochloride (HONH3+Cl-) ,Phenylhydrazine hydrochloride(C6H5NHNH3+Cl-) and semicarbazide hydrochloride(NH2CONHNH3+Cl-).The addition of sodium acetate releases free base in solution which adds on the carbonyl group as follows:
| H+ | |
C = O + : NH2OH --------> ---C---NHOH -------> C = NOH + H2O
| | |
OH
hydroxylamine oxime
| H+ | |
C = O + NH2NHC6H5 ---------> ---C---NHNHC6H5 ---------> C = NNHC6H5 + H2O
| | |
OH
phenylhydrazine phenylhydrazone
7) Addition of alcohol: Aldehydes react with anhydrous ethanol in the presence of anhydrous ethanol in the (usually dry HCl) to form acetal.The reaction taking place is.
H H H
| H+ | ROH |
R`---C = O + ROH <--------> R`---C---OR <----------> R---C---OR + H2O
| |
OH OR
hemiacetal acetal
Hemiacetals are too unstable to be isolated. Acetals are readily cleaved by acids and are stable toward base:
H H
| |
R`---C---OR + H2O ------------> R`---C = O + 2ROH
|
OR
8) Cannizzaro reaction: The reaction is shown by aldehydes containing no a hydrogen. In the presence of concentrated alkali, two molecules of an aldehyde undergo self-oxidation-and -reduction to yield a mixture of an alcohol and a salt of a carboxylic acid for example.
50 % NaOH
2HCHO ------------------> CH3OH + HCOO(-) Na(+)
methanol sodium formate
9) Aldol condensation: This reaction is shown by aldehydes or ketones containing a hydrogen.In the presence of a base, a carbon of one molecule gets attached to the carbonyl carbon of the second molecule. for example:
H H H H H H
| | | | | |
CH3---C = O H---C---C = O ---------> CH3---C---C---C = O
| | |
H OH H
aldol
Stepwise process is:
1) CH3CHO + OH(-) <--------------> H2O + (-)CH2CHO
H H
| |
2) CH3---C = O + (-)CH2CHO <--------------> CH3---C---CH2CHO
|
(-)O
H H
| |
3) CH3---C---CH2CHO + H2O <---------------> CH3---C---CH2CHO + OH(-)
| |
O OH
Distinction between Aldehydes and Ketones:
Aldehydes show the the following characteristic tests. Ketones donot show these tests.
 |
| Tollens test |
Tollens test: The Tollens reagent is an ammoniacal solution of silver nitrate.Aldehydes reduce Tollens reagent to a bright silver mirror.
 |
| Fehling`s test |
Fehling`s test: Fehling`s solution consists of an equimolar mixture of Feling`s solution. A and Fehling`s solution B.Fehling`s Solution A is CuSO4 solution and Fehling`s solution B is a mixture of Rochelte salt (sodium potassium tartarate) and sodium hydroxide. Aliphatic aldehydes (but not aromatic) reduce Fehling`s solution to red brown cuprous oxide.
 |
| Schiff`s Test |
Schiff`s Test: Schiff`s reagent is rosaniline hydrochloride solution in water decolourised with SO2.Aldehydes when warmed with Schiff`s reagent restore pink colour of the reagent.
































 Online Movies
Online Movies