 |
| Detail Carbohydrates-Chart |
Carbohydrates:
The Carbohydrates are sugar. Carbohydrates are composed of Carbon, Oxygen and Hydrogen and the Carbon atoms are normally arranged in a ring, with the oxygen and hydrogen atoms linked to them. When the 2 sugars link up, the reaction occurring expels a molecule of water and the resulting bond is called glycosidic linkage.
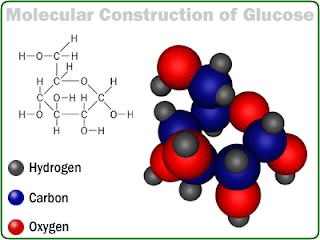 |
| Molecular Structure of Glucose |
 |
| Disaccharide |
Simple sugars like , glucose, can exist as single units, and are referred to as Mono saccharides. Glucose is the main form in which sugar is used by cells, and load levels are tightly controlled. Frequently the Mono saccharides are linked together, the resulting molecule ranging from 2 sugars or Di saccharides, example: Sucrose (Table Sugar), to long chains containing many thousands of Mono saccharides. Such complex Carbohydrates are called Polysaccharides, example: Starch.
 |
| Polysaccharides |
Glucose can be broken down (Metabolised) in either the presence (aerobically) or the absence (anaerobically) of oxygen, but the process is much more efficient when oxygen is used. During this process, energy water and CO2 are released. This family of molecule:
1) Serve as ready source of energy to fuel cellular activities.
2) Provides a integral part of the structure of DNA and RNA.
3) Can act as receptors on the cell surface, allowing the cell to recognise other molecules and cells.
 |
| Detail Lipid Chart |
Lipids:
Lipids are made up of Carbn, Hydrogen and Oxygen atoms. One group of Lipids the Phospholipids, forms an integral part of the cell membrane.
 |
| Integral Part of Cell Membrane with Lipid Bilayer |
 |
| Animated Lipid-Bilayer |
Other types of Lipids include certain Vitamines (Ex: E and K), an important groups of hormones called steroids and the fats.
 |
| honeycomb lipid Structure |
A molecule of fat consists of 3 fatty acids each linked to a molecule of glycerol, Fats are the source of energy and provide a convenient form in which to store excess energy intake. When fats are broken down they release energy. But the process is less efficient than when Carbohydrates are used, since it requires more energy for the breakdown reaction to take place. They are used in the body for:
1) Insulation.
2) Protection of body parts.
3) Energy Storage.
 |
| Detail Protein Chart |
Proteins and Amino Acids:
Amino acids always contain Carbon, Hydrogen, Oxygen and Nitrogen, and many in addition carry Sulphur. In human Bio-Chemistry, 20 Amino acids are used as the principal building blocks of protein, although there are other; for instance, there are some amino-acids used only in certain proteins and some are seen only in microbial products. Of The amino acids used in human protein synthesis, there is a basic common structure, including an amino group (NH2) a carboxyl group (COOH) and a hydrogen atom. What makes one amino acid different from next is a variable side chain. As in formation of glycosidic linkage, when two amino acids join up the reaction expels a molecule of water and the resulting bond is called a peptide bond.
 |
| Picture of Ten Amino-Acids |
 |
| Picture of Other Ten Amino-Acids |
Proteins are made from amino acids joined together, and are the main family of molecules from which the human body is built. Protein Molecules vary enormously in size, shape, chemical constituents and function. Many important groups of Biologically active substance are proteins:
1) Carrier molecules e.g. Hemoglobin.
2) Enzymes.
3) Many hormones e.g. Insulin.
4) Antibodies.
Proteins can also be used as an alternative energy source, usually in dietry inadequancy, although the process in much less efficient than when Carbohydrates or fats are broken down.
 |
| Picture of Different Structures of Different Proteins |
Primary Structure of Proteins:
A covalent bond forms between the amino group of one amino acid and the carboxyl group to another. This covalent linkage, called a peptide bond, results in a molecule called dipeptide. Three or more amino acids linked together form polypeptide chain. Which kind of amino acid follows another in the chain is always the same for all proteins of a given type. For example, the two chains making up the protein Insulin always have the sequences same. The specific sequence of amino acids in a polypeptide chain constitute the primary structure of protein.
Secondary Structure of Proteins:
The term refers to the helical or extended pattern brought about by Hydrogen bonds at regular intervals along a polypeptide chain. Some amino acids tend to favour helical pattern, other tend to favour sheetlike patterns.
 |
| An alpha-helix with hydrogen bonds (yellow dots) |
Tertiary Structure of Proteins:
Protein structure is also affected by interactions among R groups. Most helically coiled chains become further folded into some characteristic shape when one R group interacts with another R group some distance away, with the backbone itself, or with other substances present in the cell. The term tertiary structure refers to the folding that arises through interaction among R groups of a polypeptide chain.
Quaternary Structure of protein:
The 4th level of protein in architecture, results from interactions between two or more polypeptide chains in some protein.
 |
| This is a computer-generated 3D model of a Hemoglobin protein rendered as a tetra-mer with heme molecule represented in a space-filling form. |
The resulting protein can be globular, fiber like,or some combination of the two shapes e.g. Hemoglobin.
 |
| Detail Nucleotide and ATP Chart |
Nucleotides:
 |
| Closer Look Of DNA |
These are the largest molecules in the body and are built from components called nucleotides, which consists of three subunits are : A sugar ( ribose or deoxyribose) a nitrogen containing base (single ringed pyrimidine or double ringed purine) and one or more phosphate groups.
 |
| DNA - Structure |
There are three kind of nucleotides are the adenosine phosphates, the nucleotide coenzymes and the nucleic acids ( deoxyribonucleic acid or DNA ribonucleic acids or RNA).
 |
| Animated DNA |
Adenosine Tri phosphate (ATP):
ATP is nucleotide that contain Ribose (The sugar unit) adenine ( The base) and three phosphate groups attached to the Ribose.
 |
| Ball and Sticks Model of ATP |
 |
| Molecular Structure of ATP (Adenosine Tri-Phosphate) |
It is some time known as the energy currency of the body, which implies that the body has to earn (Synthesis) it before it can so end it. Many of the body`s huge number of reactions release energy e.g. the breakdown of sugars in the presence of Oxygen. The body captures energy released by this reactions, using it to make ATP from Adenosine Diphosphate (ADP). When the body needs chemical energy to fuel cellular activity, ATP releases its stored energy and a phosphate group through the splitting of a high energy phosphate bond, and reverts to ADP. The body needs Chemical energy to:
1) Drive synthetic reactions(Building Biological molecules).
2) Fuel Movements.
3) Transport substance across membrane.
























 Online Movies
Online Movies
No comments:
Post a Comment