
The Atom
is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons (except in the case of hydrogen-1, which is the only stable nuclide with no neutrons). The electrons of an atom are bound to the nucleus by the electromagnetic force.
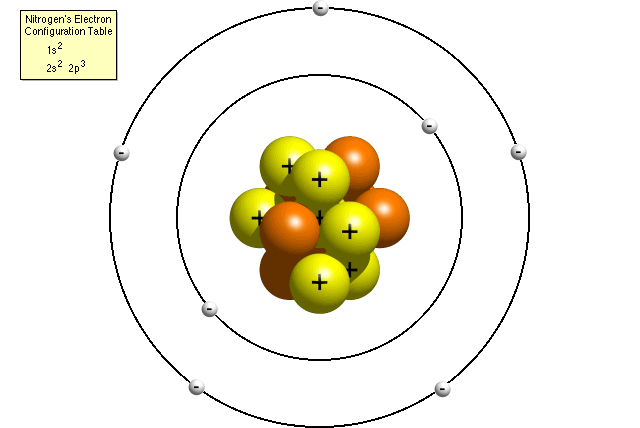 |
| Structure of Nitrogen Atom |
 |
| Atomic Structure of NaCl |
CHEMICAL BONDS:
The formation of chemical bond between atoms implies that the system consisting of these two atoms at stable internuclear distance is energy - tically more stable than two isolated atom. A general study on the reactivity of different elements revealed that noble gases have little tendency to combine with other elements. This lead to the fact that the noble gases have
2 6
stable outer configuration ns np (octet configuration) or by complete transfer of electrons from one atom to other (Ionic Bond).
 |
| Covalent Bond Within NH3 |
Covalent Bond:
A covalent bond forms when two atoms link up by sharing electrons. Each atom supplies an electron, and the pair of electrons orbits the nuclei of both atoms, holding the atom together as a molecule.The molecule of covalent compounds are held together by weak bond called Van Der Waal`s forces. Some hydrogen containing compounds, such as water, have stronger forces called hydrogen bonds between their molecules. In water, these bonds form because each oxygen atom in a water molecule is attracted to hydrogen atoms in two nearby molecules. Covalent bonds in ammonia molecule (NH3).
 |
| Double Bond Within Oxygen |
Double Bonds:
Sometimes atoms form covalent bonds by sharing two pairs of electrons. This is called a double bond. A triple covalent bond forms when atoms share three pairs of electrons. Double bond links two oxygen atoms.
 |
| Ionic Bond Within NaCl |
Ionic Bonds:
When an electron transfer from one atom to another,the atoms become charged particles called ions.The atom losing the electron becomes a positively charged ion, and the atom gaining the electron becomes a negatively charged ions opposite charges is called an ionic bond.Formation of ionic bonds in Sodium Chloride (NaCl). Sodium atom loses electron, Electron trasfers between atoms, Chlorine atom gains electron.
 |
| Different Geometrical structures form during the Formation of Co-ordinate Bonds |
Coordinate Bond:
If the pair of electrons shared between two atoms is exclusively contributed by one of the atoms, a coordinate bond is said to be formed. One of The example is:
H F
. . . .
H : N : ---------------> B : F
. . . .
H F
Another example is NH4 ion. Once The bond is formed, it is not possible to distinguish between the covalent and Coordinate bond.
There are two kinds of chemical bonds present in these cobalt(III) compounds. One is ionic (similar to the bond between Na+ and Cl- in solid NaCl) and the other is a coordination bond. An example of a coordination bond that you may have seen before is the following: F3B:NH3
The bond between B and N is a coordinate bond.
Coordinate covalent bond
A single covalent bond in which both electrons in the shared pair come from the same atom. The molecule or ion which contains the donor atom is called a ligand.
 |
| Hydrogen Bond Within The Ice-Crystal |
Hydrogen Bond:
A bond between a hydrogen atom and a highly electronegative atom of small size (e.g. F, N,O) is a polar bond in which hydrogen atom acquires a small positive charge while the electronegative atom acquire small negative charge. The Hydrogen atom can be attached to the electronegative atom of other molecule carrying a lone pair of electrons due to the characteristics, the hydrogen bond is also directional in nature.
A hydrogen bond may be intra molecular or inter molecular. In the former, the hydrogen atom is attached to the electronegative atom belonging to the same molecule where as in the latter, it is attached to the electronegative atom of some other molecules. Example are O-nitrophenol and P-nitrophenol
The hydrogen bonding affects the properties of a substance to a large Extent. For example, the boiling point of water is much more than hydrogen sulphide.
























 Online Movies
Online Movies
I would like to say that this blog really convinced me to do it! Thanks, very good post. ispace1
ReplyDeleteThe profundity of articles can without much of a stretch be felt of this blog. Exceptionally exact and straight to the check. I saw effectively the obvious reality which the creator of this blog needed to convey through his contemplations. Searching for additional. immigration lawyer ga
ReplyDeleteAbsolutely fantastic posting! Lots of useful information and inspiration, both of which we all need!Relay appreciate your work.Best Video Editing Softwares
ReplyDelete