 |
| Animated Amines |
Chemistry of Amines
1. Nomenclature and Structure of Amines
 |
| Different Types of Amines |
In the IUPAC system of nomenclature, functional groups are
normally designated in one of two ways. The presence of the function may be
indicated by a characteristic suffix and a location number. This is common for
the carbon-carbon double and triple bonds which have the respective suffixes
ene and yne. Halogens, on the other hand, do not have a suffix and are named as
substituents, for example: (CH3)2C=CHCHClCH3
is 4-chloro-2-methyl-2-pentene. If you are uncertain about the IUPAC rules for
nomenclature you should review them now.
Amines are derivatives of ammonia in which one or more of the
hydrogens has been replaced by an alkyl or aryl group. The nomenclature of
amines is complicated by the fact that several different nomenclature systems
exist, and there is no clear preference for one over the others. Furthermore,
the terms primary (1º), secondary (2º) & tertiary (3º) are used to classify
amines in a completely different manner than they were used for alcohols or
alkyl halides. When applied to amines these terms refer to the number of alkyl
(or aryl) substituents bonded to the nitrogen atom, whereas in other cases they
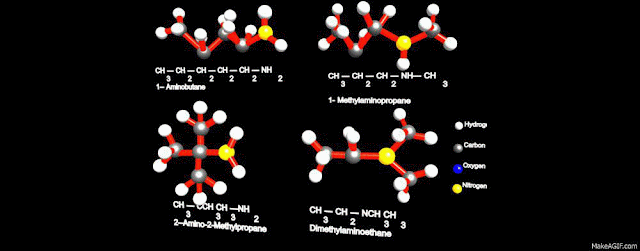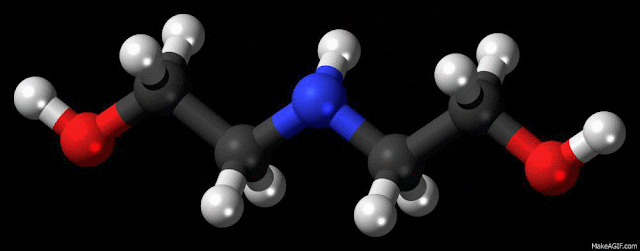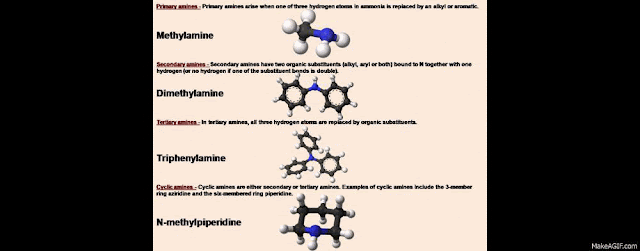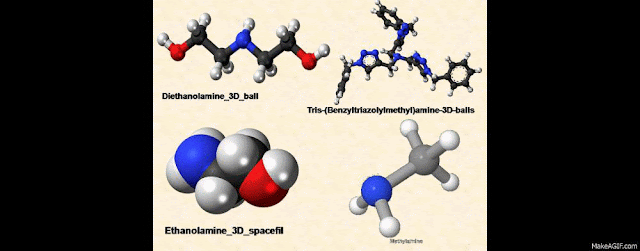
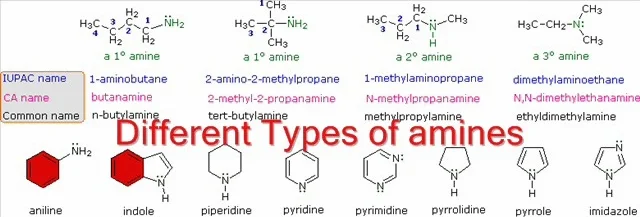
refer to the nature of an alkyl group. The four compounds shown in the top row
of the following diagram are all C4H11N isomers. The
first two are classified as 1º-amines, since only one alkyl group is bonded to
the nitrogen; however, the alkyl group is primary in the first example and
tertiary in the second. The third and fourth compounds in the row are 2º and
3º-amines respectively. A nitrogen bonded to four alkyl groups will necessarily
be positively charged, and is called a 4º-ammonium cation. For example, (CH3)4N(+)
Br(-) is tetramethylammonium bromide.
The IUPAC names are listed first and colored blue. This
system names amine functions as substituents on the largest alkyl group. The
simple -NH2 substituent found in 1º-amines is called an amino group.
For 2º and 3º-amines a compound prefix (e.g. dimethylamino in the fourth
example) includes the names of all but the root alkyl group.
The Chemical Abstract Service has adopted a nomenclature
system in which the suffix -amine is attached to the root alkyl name. For
1º-amines such as butanamine (first example) this is analogous to IUPAC alcohol
nomenclature (-ol suffix). The additional nitrogen substituents in 2º and
3º-amines are designated by the prefix N- before the group name. These CA names
are colored magenta in the diagram.
Finally, a common system for simple amines names each alkyl
substituent on nitrogen in alphabetical order, followed by the suffix -amine.
These are the names given in the last row (colored black).
Many aromatic and heterocyclic amines are known by unique common
names, the origins of which are often unknown to the chemists that use them
frequently. Since these names are not based on a rational system, it is
necessary to memorize them. There is a systematic nomenclature of heterocyclic
compounds, but it will not be discussed here
 |
| Different Types of Amines |
Classes of amines:
Amines are organized into four subcategories:
 |
| Different Classes Of Amines |
Primary amines - Primary
amines arise when one of three hydrogen atoms in ammonia is replaced by an
alkyl or aromatic. Important primary alkyl amines include methylamine,
ethanolamine (2-aminoethanol), and the buffering agent tris, while primary
aromatic amines include aniline.
Secondary amines - Secondary
amines have two organic substituents (alkyl, aryl or both) bound to N together
with one hydrogen (or no hydrogen if one of the substituent bonds is double).
Important representatives include dimethylamine and methylethanolamine, while
an example of an aromatic amine would be diphenylamine.
Tertiary amines - In tertiary amines, all three
hydrogen atoms are replaced by organic substituents. Examples include
trimethylamine, which has a distinctively fishy smell or triphenylamine.
Cyclic amines - Cyclic amines are either
secondary or tertiary amines. Examples of cyclic amines include the 3-member
ring aziridine and the six-membered ring piperidine. N-methylpiperidine and
N-phenylpiperidine are examples of cyclic tertiary amines.
It is also possible to have four organic substituents on the
nitrogen. These species are not amines but are quaternary ammonium cations and
have a charged nitrogen center. Quaternary ammonium salts exist with many kinds
of anions.
 |
| Different Types of Amines |
A Structure Formula
Relationship
Recall that the molecular formula of a hydrocarbon (CnHm)
provides information about the number of rings and/or double bonds that must be
present in its structural formula. In the formula shown below a triple bond is
counted as two double bonds.
This molecular formula analysis may be extended beyond
hydrocarbons by a few simple corrections. These are illustrated by the examples
in the table above, taken from the previous list of naturally occuring amines.
• The presence of oxygen does not alter the relationship.
• All halogens present in the molecular formula must be replaced
by hydrogen.
• Each nitrogen in the formula must be replaced by a CH moiety
 |
| Amine Producing Processes |
Properties of Amines
1. Boiling Point and Water Solubility
It is instructive to compare the boiling points and water
solubility of amines with those of corresponding alcohols and ethers. The
dominant factor here is hydrogen bonding and the first table below documents
the powerful intermolecular attraction that results from -O-H---O- hydrogen
bonding in alcohols (light blue columns). Corresponding -N-H---N- hydrogen
bonding is weaker, as the lower boiling boints of similarly sized amines (light
green columns) demonstrate. Alkanes provide reference compounds in which
hydrogen bonding is not possible, and the increase in boiling point for
equivalent 1º-amines is roughly half the increase observed for equivalent
alcohols.
The
second table illustrates differences associated with isomeric 1º, 2º &
3º-amines, as well as the influence of chain branching. Since 1º-amines have
two hydrogens available for hydrogen bonding, we expect them to have higher
boiling points than isomeric 2º-amines, which in turn should boil higher than
isomeric 3º-amines (no hydrogen bonding). Indeed, 3º-amines have boiling points
similar to equivalent sized ethers; and in all but the smallest compounds,
corresponding ethers, 3º-amines and alkanes have similar boiling points. In the
examples shown here, it is further demonstrated that chain branching reduces
boiling points by 10 to 15 ºC.
The water
solubility of 1º and 2º-amines is similar to that of comparable alcohols. As
expected, the water solubility of 3º-amines and ethers is also similar. These
comparisons, however, are valid only for pure compounds in neutral water. The
basicity of amines (next section) allows them to be dissolved in dilute mineral
acid solutions, and this property facilitates their separation from neutral
compounds such as alcohols and hydrocarbons by partitioning between the phases
of non-miscible solvents
2. Basicity of Amines
A review of basic acid-base concept should be helpful to the
following discussion. Like ammonia, most amines are Brønsted and Lewis bases,
but their base strength can be changed enormously by substituents. It is common
to compare basicities quantitatively by using the pK`sof their conjugate acids
rather than their pKb's. Since pKa + pKb = 14,
the higher the pKa the stronger the base, in contrast to the usual
inverse relationship of pKa with acidity. Most simple alkyl amines
have pKa's in the range 9.5 to 11.0, and their water solutions are
basic (have a pH of 11 to 12, depending on concentration). The first four compounds
in the following table, including ammonia, fall into that category.
The last five compounds (colored cells) are significantly weaker
bases as a consequence of three factors. The first of these is the
hybridization of the nitrogen. In pyridine the nitrogen is sp2
hybridized, and in nitriles (last entry) an sp hybrid nitrogen is part of the
triple bond. In each of these compounds (shaded red) the non-bonding electron
pair is localized on the nitrogen atom, but increasing s-character brings it
closer to the nitrogen nucleus, reducing its tendency to bond to a proton.
Secondly, aniline and p-nitroaniline (first two green shaded
structures) are weaker bases due to delocalization of the nitrogen non-bonding
electron pair into the aromatic ring (and the nitro substituent). This is the
same delocalization that results in activation of bezene ring toward
electrophilic substitutionThe following resonance equations, which are similar
to those used to explain the enhanced acidity of orthro and para-nitrophoenols
illustrate electron pair delocalization in p-nitroaniline. Indeed, aniline is a
weaker base than cyclohexyl amine by roughly a million fold, the same factor by
which phenol is a stronger acid than cyclohexanol. This electron pair
delocalization is accompanied by a degree of rehybridization of the amino
nitrogen atom, but the electron pair delocalization is probably the major
factor in the reduced basicity of these compounds. A similar electron pair
delocalization is responsible for the very low basicity (and nucleophilic
reactivity) of amide nitrogen atoms (last green shaded structure). This feature
was instrumental in moderating th e influence of amine substituents on aromatic
ring substitution, and will be discussed further in the section devoted to
carboxylic acid derivatives.
3. Acidity of Amines
We normally think of amines as bases, but it must be remembered
that 1º and 2º-amines are also very weak acids ammonia has a pKa =34 In this
respect it should be noted that pKa is being used as a measure of
the acidity of the amine itself rather than its conjugate acid, as in the
previous section. For ammonia this is expressed by the following hypothetical
equation:
NH3
+ H2O ____> NH2(-) + H2O-H(+)
The same
factors that decreased the basicity of amines increase their acidity. This is
illustrated by the following examples, which are shown in order of increasing
acidity. It should be noted that the first four examples have the same order
and degree of increased acidity as they exhibited decreased basicity in the
previous table. The first compound is a typical 2º-amine, and the three next to
it are characterized by varying degrees of nitrogen electron pair
delocalization. The last two compounds (shaded blue) show the influence of
adjacent sulfonyl and carbonyl groups on N-H acidity. From previous discussion
it should be clear that the basicity of these nitrogens is correspondingly
reduced.
The acids
shown here may be converted to their conjugate bases by reaction with bases
derived from weaker acids (stronger bases). Three examples of such reactions
are shown below, with the acidic hydrogen colored red in each case. For
complete conversion to the conjugate base, as shown, a reagent base roughly a
million times stronger is required.
|
C6H5SO2NH2
+ KOH ----> C6H5SO2NH(-) K(+)
+ H2O
|
a
sulfonamide base
|
|
(CH3)3COH
+ NaH ------> (CH3)3CO(-) Na(+)
+ H2
|
an
alkoxide base
|
|
(C2H5)2NH
+ C4H9Li ----->(C2H5)2N(-)
Li(+) + C4H10
|
an amide
base
|
Amine Reactions
1. Electrophilic Substitution at Nitrogen
 |
| Reactions of Amines |
Ammonia and many amines are not only bases in the Brønsted sense,
they are also nucleophiles that bond to and form products with a variety of
electrophiles. A general equation for such electrophilic substitution of
nitrogen is:
|
2 R2ÑH
+ E(+) ------> R2NHE(+) ----------> R2ÑE
+ H(+) (bonded to a base)
|
A list of
some electrophiles that are known to react with amines is shown here. In each
case the electrophilic atom or site is colored red.
It is instructive to examine these nitrogen substitution
reactions, using the common alkyl halide class of electrophiles. Thus, reaction
of a primary alkyl bromide with a large excess of ammonia yields the
corresponding 1º-amine, presumably by a SN2 mechanism. The hydrogen
bromide produced in the reaction combines with some of the excess ammonia,
giving ammonium bromide as a by-product. Water does not normally react with
1º-alkyl halides to give alcohols, so the enhanced nucleophilicity of nitrogen
relative to oxygen is clearly demonstrated.
|
2 RCH2Br
+ NH3 (large excess) -------> RCH2NH2 +
NH4(+) Br(-)
|
It
follows that simple amines should also be more nucleophilic than their alcohol
or ether equivalents. If, for example, we wish to carry out a SN2
reaction of an alcohol with an alkyl halide to produce an ether The Wiliamson
sysnthesis it is necessary to convert the weakly nucleophilic alcohol to its
more nucleophilic conjugate base for the reaction to occur. In contrast, amines
react with alkyl halides directly to give N-alkylated products. Since this
reaction produces HBr as a co-product, hydrobromide salts of the alkylated
amine or unreacted starting amine (in equilibrium) will also be formed.
|
2 RNH2 + C2H5Br
--------> RNHC2H5 + RNH3(+) Br(-)
<---------->RNH2C2H5(+) Br(-)
+ RNH2
|
Unfortunately,
the direct alkylation of 1º or 2º-amines to give a more substituted product
does not proceed cleanly. If a 1:1 ratio of amine to alkyl halide is used, only
50% of the amine will react because the remaining amine will be tied up as an
ammonium halide salt (remember that one equivalent of the strong acid HX is
produced). If a 2:1 ratio of amine to alkylating agent is used, as in the above
equation, the HX issue is solved, but another problem arises. Both the starting
amine and the product amine are nucleophiles. Consequently, once the reaction
has started, the product amine competes with the starting material in the later
stages of alkylation, and some higher alkylated products are also formed. Even
3º-amines may be alkylated to form quaternary (4º) ammonium salts. When
tetraalkyl ammonium salts are desired, as shown in the following example,
Hünig's base may be used to scavange the HI produced in the three SN2
reactions. Steric hindrance prevents this 3º-amine (Hünig's base) from being
methylated.
|
C6H5NH2
+ 3 CH3I + Hünig's base ------> C6H5N(CH3)3(+)
I(-) + HI salt of Hünig's base
|
2. Preparation of 1º-Amines
Although direct alkylation of ammonia by alkyl halides leads to 1º-amines,
alternative procedures are preferred in many cases. These methods require two
steps, but they provide pure product, usually in good yield. The general
strategy is to first form a carbon-nitrogen bond by reacting a nitrogen
nucleophile with a carbon electrophile. The following table lists several
general examples of this strategy in the rough order of decreasing
nucleophilicity of the nitrogen reagent. In the second step, extraneous
nitrogen substituents that may have facilitated this bonding are removed to
give the amine product.
A specific example of each general class is provided in the
diagram below. In the first two, an anionic nitrogen species undergoes a SN2
reaction with a modestly electrophilic alkyl halide reactant. For example #2 an
acidic phthalimide derivative of ammonia has been substituted for the
sulfonamide analog listed in the table. The principle is the same for the two
cases, as will be noted later. Example #3 is similar in nature, but extends the
carbon system by a methylene group (CH2). In all three of these methods
3º-alkyl halides cannot be used because the major reaction path is an E2
elimination.
3. Preparation of 2º &
3º-Amines
Of the six methods described above, three are suitable for the
preparation of 2º and/or 3º-amines. These are:
(i) Alkylation of the sulfonamide derivative of a 1º-amine. Gives
2º-amines.
(ii) Reduction of alkyl imines and dialkyl iminium salts. Gives 2º
& 3º-amines.
(iii) Reduction of amide derivatives of 1º & 2º-amines. Gives 2º
& 3º-amines.
Examples showing the application of these methods to the
preparation of specific amines are shown in the following diagram. The
sulfonamide procedure used in the first example is similar in concept to the
phthalimide example #2 presented in the previous diagram. In both cases the
acidity of the nitrogen reactant (ammonia or amine) is greatly enhanced by
conversion to an imide or sulfonamide derivative. The nucleophilic conjugate
base of this acidic nitrogen species is then prepared by treatment with sodium
or potassium hydroxide, and this undergoes a SN2 reaction with a 1º
or 2º-alkyl halide. Finally, the activating group is removed by hydrolysis
(phthalimide) or reductive cleavage (sulfonamide) to give the desired amine.
The phthalimide method is only useful for preparing 1º-amines, whereas the
sulfonamide procedure may be used to make either 1º or 2º-amines.
































 Online Movies
Online Movies
We also provide analytical services and laboratory services to our customers. TTPA
ReplyDeletenice content about amines
ReplyDeletekeep it up
Looking for reliable academic help? With Sample Assignment UK, you can pay someone to do your assignment stress-free. Their team of experts ensures top-notch quality and timely delivery. Visit their website now for professional assistance with your assignments.
ReplyDelete