 |
| Animated Alcohols |
 |
| Alcohols |
 |
| ROH |
ALCOHOLS:
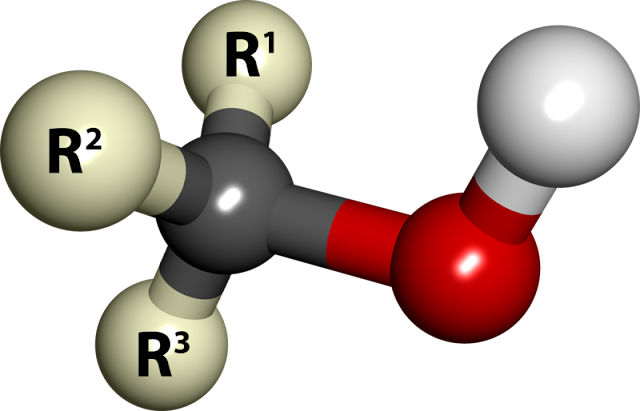 |
| 3D Picture of Alcohol |
Alcohols are the family of compounds that contain one or more hydroxyl (-OH) groups.
After the nature of the hydrocarbons radical (the radical connected to the -OH group), you can distinguish several types of alcohol:
- if the radical derives from a saturated hydrocarbon (acyclic or cyclic), the respective alcohol is a saturated alcohol (for example: ethanol CH3-CH2-OH)
- if the radical has a double bound, the respective alcohol is a unsaturated alcohol (for example: allelic alcohol CH2=CH-CH2OH)
 |
| Allyl-alcohol-3D-balls |
- if the radical contains an aromatic nucleus, the respective alcohol is a aromatic alcohol (for example: benzilic alcohol C6H6-CH2OH)
 |
| Benzyl-alcohol-3D-balls |
 |
| Benzyl-alcohol-3D |
After the number of hydroxyl groups in the molecule, you can differentiate 2 types of alcohols: mono hydroxyls (for example ethanol C2H5OH) and poly hydroxyls (for example: propane trill CH2OH-CHOH-CH2OH). The alcohols containing 2 hydroxyl groups
 |
| Phenethyl-alcohol-3D-balls |
connected by different carbon atoms are called glycols, for example: CH2OH-CH2OH
The classification of mono hydroxyl alcohols
The mono hydroxyl alcohols can be classified after the primary / secondary / tertiary carbon atom connected to the hydroxyl, in:
- primary alcohols
- secondary alcohols
- tertiary alcohols
For example, ethanol CH3-CH2-OH, is called a primary alcohol; 2-propanol CH3-CH(OH)-CH3 is a secondary alcohol and 2-methyl-2-propanol CH3-C(CH3)OH-CH3 is a tertiary alcohol.
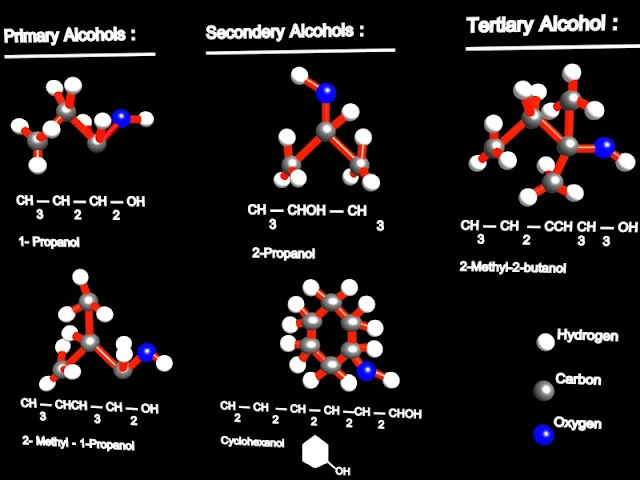 |
| 3D Pictures of Different Alcohols |
1) Oxymercuration-Demercuration Process: Alkenes react with mercury acetate in the presence of water to give hydroxymercurial compounds which on reduction with sodium borohydride (NaBH4) yield alcohol,the net result is the addition of H2O to the double bond and the product obtained is in agreement with Markovnicov`s rule.For example.
CH3CH = CH2 + H2O + Hg(OAc)2 -------> CH3---CH---CH2
| |
OH HgOAc
|
CH3---CH--- CH3
|
OH
2) Hydroboration-Oxidation Process: Alkenes undergo hydroboration with diborane to yield alkylboranes which on oxidation with alkaline H2O2 give alcohols.The net result is the addition of H2O to the double bond and the rpoduct obtained is in accorance with the anti-Markovnikov`s rule. For example,
CH3CH = CH2 + BH3 --------> CH3CH2CH2BH2
| H2O2, OH(-)
|
CH3CH2CH2OH
n-propyl alcohol
3) Grignard Synthesis of Alcohol: Reaction of aldehyde or ketone with RMgX produces alcohol.
H H
| - + |
H---C=O + R---MgX --------> H---C---OMgX -------------> H---C---OH
| | |
H R R
1` alcohol
H H
| - + |
H---C=O + R---MgX-------> R`---C---OMgX ------------> R`---C---OH
| |
R R
2` alcohol
 |
| Grignard Synthesis of Alcohol |
4) Reaction of ethylene oxide with RMgX:
H2O
CH2---CH2 + RMgX -------------> RCH2---CH2OMgX ----------------> RCH2CH2OH
\ /
\ /
O
5) Reactions of lithium acetylides or alkyl Grignard reagents with aldehyde or ketone:
R R R
_ | _ | H(+) _|
HC = Cli + O = C---R` --------> HC = C---C---R` -------> HC = C---C---R`
| |
OLi OH
R R R
_ | _| |
HC = CMgBr + O = C---R` --------> HC = C---CR` -------> HC = C---C---R`
| |
OMgBr OH
6) Reduction of Aldehyde and Ketones: Sodium borohydride, NaBH4 is the convenient reagent to carry out the reduction of aldehydes or ketones into alcohol.
1. NaBH4 in alcohol
RCHO --------------------------------------> RCH2OH
2. H3O(+)
LiAlH4 inether can also be used for reduction.It is Particularly useful for reducing a, b- Unsaturated ketones. The reduction of such a ketone gives a mixture of both unsaturated and saturated alcohols. For example:
Physical properties:
Due to the hydrogen bonding, alcohols have higher boiling points than their structural isomers. The boiling point decreases with increase in branching,i.e. the order of boiling point is primary > secondary > tertiary.
Chemical properties:
The general formula of simple alcohol is ROH.The reaction shown by alcohols may be classified into two categories, namely, R-----OH bond cleavage and RO-----H bond cleavage.
Reaction exibiting R----------OH bond cleavage.
1) Reaction with hydrogen halides:
R---OH + HX ------> RX + H2O
The mechanism is:
R---OH <----------------> R---OH2 --------> R---X + H2O
As such ----OH is a poor leaving group.But its protonation convers into a good leaving group.There is formation of carbocation as the intermediate and thus the reaction may show rearrangement. The following are the reactivities of HX and ROH.
HI > HBr > HCl
allyl, benzyl > 3` > 2` > 1`
The reagents, used are concentrated HBr or NaBr + concentrated H2SO4, HCl + ZnCl2 and concentrated HCl.
2) dehydration:
CH3---CH2---CH2---CH2OH---------> CH3CH = CHCH3 + CH3CH2CH = CH2
2-butane 1-butane
(main product)
Primary alcohol is dehydrated using concentrated H2SO4. The secondary and teriary alcohols are dehydrated using
dilute H2SO4 to avoid the polymerization of alkenes. Saytzeff`s rules is applicatble so as to get more subtituted alkene.
3) Reaction with phosphorous trihalides:
3R---OH + PX3 -------> 3R---X + H3PO3
(PX3 = PBr3, PI3)
4) Reaction with active Metals:
RO---H + M ---------> ROM(+) + 1/2 H2
reactivity of alcohol CH3OH>1`>2`>3`
The above reaction shows alcohol as an acid.It is worth comparing the acid strength of alcohol with other species based on the following reactions.
RO(-) Na(+) + H---OH -------------> Na(+) OH(-) + RO---H
stronger base Stronger acid weaker base weaker acid
_ _
HC = CNa + RO---H --------------> RO(-)Na(+) + HC = C---H
stronger base stronger acid weaker base weaker acid
H2N(-)Na(+) + RO(-)H ------------->RONa + H2N---H
stronger base stonger acid weaker base weaker acid
ROH + R`MgX ------------> R`H + Mg(OR)X
stronger acid weaker acid
Thus the relative order of acidity is:
_
H2O > ROH > HC = CH > NH3 >RH
The relative order of basicity follows the reverse order, i.e.
_
OH(-) < OR(-) < HC = C(-) < NH2(-) < R(-)
5) Reaction with carboxylic acid: This results in the formation of an estar.
O O
|| H(+) ||
CH3---C---OH + H---OC2H5 <------------------> CH3---C---OC2H5 + H2O
(ethyl acetate)
Instead of acetic acid, acetic anhydride or acetyl chloride can also be used.
6) Oxidation of alcohols: The oxidation of an alcohol involves the loss of one or more a - hydrogens. 1` alcohol is changed to an aldehyde by using the reagent pyridinium chlorochromate (C5H5NH(+) CrO3Cl(-)) formed by the reaction between chromic acid and pyridinium chloride. For example,
H
C5H5NH(+)CrO3Cl(-) |
CH3CH2CH2OH ---------------------------------> CH3CH2---C = O
1-propanol propanal
1` alcohol is directly converted into a carboxylic acid by the use of potassium permanganate. for example:
O
KMO4 ||
CH3CH2CH2OH------------------------------> CH3CH2---C = OH
1-propanol propanoic acid
2` alcohol is changed into a ketone by the use of potassium dichromate or CrO3 in glacial acetic acid or CrO3 in pyridine.For example:
CH3CHOH ---------------------> CH3C = O
| |
CH3 CH3
isopropyl alcohol acetone
Characterization of Alcohols:
1) Oxidation Method:
[O] [O]
1` alcohol -------------------> aldehyde--------------> carboxylic acid
[O]
2` alcohol --------------------> ketone
[O]
3` alcohol ----------------------> no reaction
 |
| Oxidation Method |
However, drastic oxidation will convert ketone (formed from 2` alcohol) and 3` alcohol into carboxylic acid containing fewer carbon atoms. When the vapours of alcohols are passed over hot metallic Cu at 570 K, limited oxidation takes place.
[O]
1` alcohol: CH3CH2OH ----------------> CH3CHO + H2O
[O]
2` alcohol: CH3CHCH3 -----------------> CH3COCH3 + H2O
|
OH
[O]
3` alcohol: (CH3)3COH -------------------> CH3C = CH2 + H2O
|
CH3
 |
| Alcohol- Aldehyde |
2) Victor-Meyer Method: The alcohols are treated with P4 and I2 to form an alkyliodide. This on treatment with AgNO2 acidified with HCl and then alkaline with NaOH produces different colouration.
 |
| Victor-Meyer Method: |
3) Lucas Test: Lucas reagent is a mixture of concentrated hydrochloric acid and zinc chloride, it converts alcohol into chloride and the formation of the latter is indicated by the appearance of cloudiness which marks the separation of chloride from the solution:
 |
| Lucas Test Reactions |
A tertiary alcohol reacts immediately, a secondery alcohol reacts within five minutes and a primary alcohol does not react appreciably at room temperature.
Allyl alcohol reacts as rapidly as tertiary alcohols but in remains in the solution.
Characteristic Test of CH3CO------Group: An alcohol of the type R---CH---OH is oxidized to
||
O
R---C---CH3 which give iodoform
|
CH3
test
 |
| Lucas Test Pictures |
What Is Fermentation:
 |
| Fermentation Reaction |
A solution of glucose in water remains unchanged indefinitely.If however, to this solution some yeast is added, after sometime the whole liquid begins to froth and looks like boiling.On Testing it is found that glucose has been converted to ethyl alcohol and the boiling appearance is, in fact, due to brisk evolution of carbon dioxide during the reaction.
Yeast
C6H12O6 --------------------> 2CH3CH2OH + 2CO2
Glucose Ethyl Alcohol
 |
| 3D Picture of Fermentation |
This process of conversion of sugers to ethyl alcohol under the influence of yeast was known since the remotet times and was named Fermentation (Fever = to boil).
Yeast is single celled living plant.It grows and multiplies rapidly under suitable conditions. It is believed that some " Non-Living" complex organic compounds present in yeast are responsible for change.These complex compounds bring about fermentation by playing the role of a catalyst.
Raw Materials: In alcoholic fermentation cane sugar or glucose are the fermenting materials. Therefore any natural product which contains these sugars or can be easily converted into them, is a potential source of ethyl alcohol. The raw materials for alcohol industry are:
1) Substance containing FERMENTABLE SUGARS. Examples are : cane Juice, beets, dates, molasses and fruit juices.
2) Substances containing STARCH. Examples are : potatoes, rice, barley, and maize.
 |
| Fermentation Reaction Machine |
 |
| Bad Effects of Alcohol in Human Body |
























 Online Movies
Online Movies
No comments:
Post a Comment