 |
| Electrochemistry Post |
 |
| Volta Cell Page in Chemistry-World Software |
ELECTROCHEMICAL CELLS:
 |
| Volta Cell |
Galvanic and Electrolytic Cells
Oxidation-reduction or redox-reaction take place in electrochemical cells. There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; nonspontaneous reactions occur in electrolytic cells. Both types of cells contain electrodes where the oxidation and reduction reactions occur. Oxidation occurs at the electrode termed the anode and reduction occurs at the electrode called the cathode.
 |
| Electron Flow from Anode to Cathode in This Cell |
Electrodes & Charge
The anode of an electrolytic cell is positive (cathode is negative), since the anode attracts anions from the solution. However, the anode of a galvanic cell is negatively charged, since the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The cathode of a galvanic cell is its positive terminal. In both galvanic and electrolytic cells, oxidation takes place at the anode and electrons flow from the anode to the cathode.
 |
| All Working Principles in Volta Cell |
Galvanic or Voltaic Cells
The redox reaction in a galvanic cell is a spontaneous reaction. For this reason, galvanic cells are commonly used as batteries. Galvanic cell reactions supply energy which is used to perform work. The energy is harnessed by situating the oxidation and reduction reactions in separate containers, joined by an apparatus that allows electrons to flow. A common galvanic cell is the Daniell cell. In galvanic cell, current is generated as a result of a spontaneous chemical reaction that occurs in the cell.The main characteristics of galvanic cells are given in below
Anode Cathode
Sign Positive as the electrons are consumed Negative as the electrons are released at this electrodes at this electrodes.
Half-reaction Oxidation Reduction
Direction of electron movement Into the cell. Out of cell.
Thus electrons are generated at the anode because of the oxidation reaction. As the electrons are negatively charged species, the anode becomes the negative end of the cell . The generated electrons are moved through the external wire to the outer electrode (cathode) where these are consumed due to the reduction reaction.This electrode is deficient in electrons and is therefore , the positive terminal of the cell.
Cell reaction:
A cell can be briefly represented by following the rules given below.
1)The separation of two phases is shown by a vertical line.
2) The various materials present in the same phase are shown together with the help of commas.
3) the two half cells are joined with the help of double vertical lines
4) Significant features of the substances such as pressure of the gas concentration of ions , etc. are indicated in brackets, drawn immediately after writing the substance
According to the recommended convention, if a cell is written in brief , the left half cell (LHC) is considered as the negative terminal and the right half cell(RHC) as the positive terminal .Thus , the oxidation reaction occurs at the left electrode and the reduction reaction at the right electrode. A few examples of illustrating the above convention are described below.
1. Zn | Zn(2+)(aq) || Cu(2+)(aq) | Cu
LHC: Oxidation Zn ------> Zn(2+) + 2e(-)
RHC: Reduction Cu(2+)(aq) + 2e(-) -------> Cu
---------------------------------------------------------
Overall reaction Zn + Cu(2+)(aq) -----> Zn(2+)(aq) + cu
-----------------------------------------------------------
2) Pt | H2(g) | H(+)(aq) || Zn(2+)(aq) | Zn
LHC: Oxidation H2(g)------> 2H(+)(aq) + 2e(-)
RHC: Reduction Zn(2+)(aq) + 2e(-) -------> Zn
-----------------------------------------------------------
Overall reaction H2(g) +Zn(2+)(aq) ----->2H(+)(aq) + Zn
-------------------------------------------------
 |
| Electrolytic Cell Page in My Chemistry-World Software |
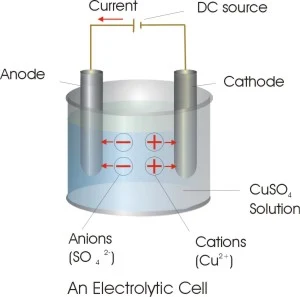
Electrolytic Cells
The redox reaction in an electrolytic cell is nonspontaneous. Electrical energy is required to induce the electrolysis reaction. An example of an electrolytic cell is shown below, in which molten NaCl is electrolyzed to form liquid sodium and chlorine gas.
 |
| An Electrolytic Cell |
The sodium ions migrate toward the cathode, where they are reduced to sodium metal. Similarly, chloride ions migrate to the anode and are oxided to form chlorine gas. This type of cell is used to produce sodium and chlorine. The chlorine gas can be collected surrounding the cell. The sodium metal is less dense than the molten salt and is removed as it floats to the top of the reaction container.
 |
| All Working Principle of Electrolytic Cell |
The phenomenon of electrolysis involves the breaking of electrolysis when an electric current is passed through them.The apparatus used to carry out the electrolysis is known as electrolytic cell. The main characteristics of an electrolytic cell are described below.
Anode Cathode
Sign Negative as it is attached to the negative Positive as it is attached to the
end of a external battery. positive end of the external
battery.
Direction
of electron Into the cell. Out of the cell.
movement
Ions attracted
within the cell Cations Anions
Half-reaction reduction oxidation
Thus electrons are received from the negative end of the external battery by the negative electrode of the cell . These are used up in the reduction reaction at this electrode. The number of electrons received at the negative end are given back to the positive end of the external battery from the positive electrode of the cell where electrons are released as a result of oxidation reaction.Within the cell , current is carried by the movement of ions;cation towards negative electrodes (called the cathode) and anion towards the positive electrode (called the anode)
 |
| Electrolytic Cell |
The electrolysis of molten salts produces substances whic are characteristic of salt.When certain aqueous salt solutions are electrolyzed , however , water is involved in the electrode reactions rather than ions derived from the solute. Hence , current carrying ions are not necessarily discharged at theelectrodes.The reaction involving the electrolysis of water as follows
Oxidation : 2H2O ------> O2 + 4H(+) + 4e
Reduction: 2H2O + 2e(-) --------> H2 + 2OH(-)
The following rules are helpful, while predicting the electrode rea\ction during the electrolysis of aqueous salt solution.
 |
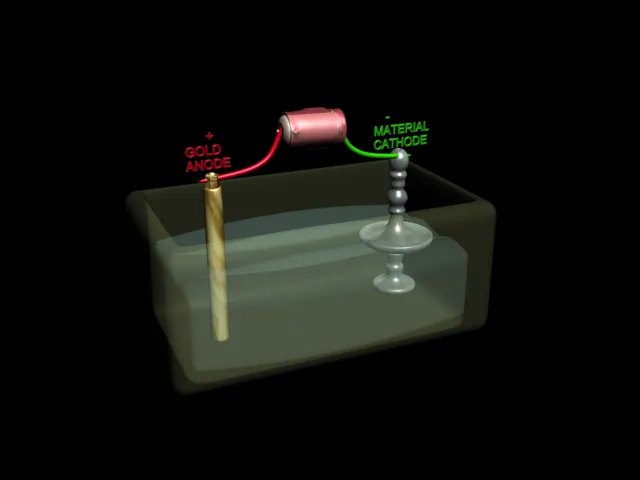
| Electrolytic Cell : Electroplating of Gold Metal on Candle Stand |
1. The species having a large reduction potential is preferentially reduced at the cathode.
2. The reduction potential of water is - 0.828 V. Water is reduced in preference to the cations having more negative potential than -0.828 V. Examples are Li(+), K(+), Na(+), Mg(2+) and Al(3+) ions.
3. Cations having less negative potential than -0.828 V are reduced in preference to water. Examples are Fe(2+), Sn(2+), Pb (2+), Ag(+) and Cu(2+) ions.
4. In acidic medium with larger concentrations of H(+) ions, The cathodic reaction involves the reduction of H(+) ions, i.e. 2H(+) + 2e(-) ------> H2.
5. At the anode , the species having maximum reduction potential is formed from the oxidation of corresponding oxidizable species.
6. If the anion is not oxidized readily, water is oxidized liberating O2.Examples are SO4(2-) and NO3(-) ions.
7. If the solution is highly alkaline, the anode process is
4OH(-)------> O2 + 2H2O + 4e(-)
8. Chloride liberates Cl2 but O2 is also liberated as the solution is diluted. This is due to the phenomenon of over voltage.
9. Metallic anodes more reactive than platinum tend to pass into solution instead of O2 being produced.
10. Soluble iodides always liberate iodine at the anode.
 |
| Electrolysis |
Electrolysis
Electrolysis is used for coating metal objects and parts with a thin layer of a different metal.Here are some objects that have been coated in this way.The part to be coated is hung in a bath of electrolyte and made the cathode of a cell. The anode is made of the metal to be deposited .When an electric current is passed , the object is covered with a layer of this metal.This is called electroplating .Usually objects are electroplated with expensive metals such as gold , silver, copper, and chromium. Car bumpers are made of steel and covered with chromium to make them shiny.It would be too expensive to make the whole bumper out of the chromium.
 |
| Faraday`s Experiment |
 |
| Faraday`s Law |
1) The mass of a chemical substance involved at an electrodes is directly proportional to the quantity of electricity (expressed in coulombs, which is product of current in ampheres and time in seconds) passed through the cell.
2) The masses of different substances produced by a given quantity of electricity are proportinal to the equivalent mass( i.e. mass corresponding to a total of unit charge on each ion of the substance) of the substance.
 |
| All Working Principles of Car Battery |
























 Online Movies
Online Movies
Our electricity problem is sorted out by the batteries and which works on electrochemical mechanism.
ReplyDeleteElectroplating in India - shivam enterprises is provider for best service for electroplating in india & electroplating in Gurgaon. For more detail visit our website http://www.electroplatingindia.in
ReplyDeleteElectroplating in India - shivam enterprises is provider for best service for electroplating in india & electroplating in Gurgaon. For more detail visit our website http://www.electroplatingindia.in
ReplyDeleteThe analytical chemistry literature lacks an adequate automatic titration method for the monitoring of chromic acid in chromium plating solutions and the monitoring of phosphoric and sulfuric acids.galvanische behandlungen
ReplyDeleteInitial You got a awesome blog .I determination be involved in plus uniform minutes. i view you got truly very functional matters , i determination be always checking your blog blesss. galvanisieren kunststoff
ReplyDeleteThanks for the blog filled with so many information. Stopping by your blog helped me to get what I was looking for. Now my task has become as easy as ABC. EZ Battery Reconditioning Review
ReplyDelete