 |
| Introduction picture of Elementary Particles |
ELEMENTARY PARTICLES:
Matter consists of groups of atoms. In the early nineteenth century John Dalton proposed that atoms could not be divided into anything smaller. This is now known to be incorrect .Atoms are composed of a central nucleus around which move electrons.The nucleus contains protons and neutrons are composed of even smaller particles called quarks.Quarks and electrons are elementary particles. This means that they cannot be divided into smaller particles. They are the basic unit of matter.
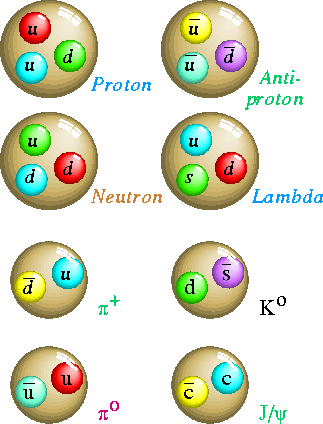 |
| Picture of Quarks |
Although these particles cannot subdivided, Some can change or decay into other elementary particles . They are therefore unstable , and only last for a certain time.
PHOTONS AND NEUTRINOS:
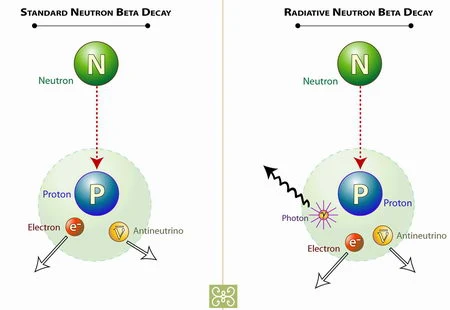 |
| Picture of Neutron |
When the neutron is outside the nucleus it is unstable .It lasts about 15 minutes then changes into a proton , an electron,and another elementary particle called the neutrino. These three particles are all stable and do not decay into other particles. The neutrino is a strange particle. It has no mass and no charge .It is a tiny bundle of energy. It is therefore difficult to detect,and was only discovered in 1956.
The photon is an elementary particle . Like the neutrinos , it is stable , has no mass or charge and is a bundle of energy. Radio waves, light and X-rays can all be thought of as a stream of photons. These types of radiation only differ in the amounts of the energies of the photons.
 |
| Charges of Sub-Atomic Particles |
There are large number of elementary particles apart from those already mentioned. They are all unstable, Some of them decaying in minute fractions of a second . Elementary particles can react together to produce other particles, but charge reaction can only occur if certain laws are obeyed. The total charge of a particles reacting together(or decaying) must equal the total charge of the particles formed. When a neutron decays, its zero charge is balanced by the positive charge of proton and the equal but negative charge of electron .This is called the conservation of charge .Many other properties
of the particles reacting together must also be conserved.
PARTICLE ACCELERATORS:
The properties and reactions of the elementary particles are studied by nuclear physicists. They use particle accelerators to produce beams of high energy particles. The first of these machines were built in 1930s. Since then machines have been built that produce particles which have an energy many thousands of times greater than those of original accelerators. Some of these machines are very large . The Fermi lab machine near Chicago is a ring some 6.5 Km across. These very energetic beam of particles are made to collide with atoms, which are usually hydrogen. This happens in a bubble chamber. When charged particles move through the bubble chamber a trace is produced.This trace is photographed and type of trace tells the scientists what type of particle caused it .They use this information to built up a picture of the structure of matter.
Animation on Cosmic rays particles from outer space .
The particles are grouped by their mass. The names of the groups are from the Greeks words of light, medium and heavy .The light particles are called leptons and include the electron. The medium mass particle are called mesons. The heavy particles are the baryons of which the most notable are the proton and the neutron.
 |
| Cosmic rays and particles from outer space |
Cosmic rays from outer space . This high energy radiation consists mainly of protons and some alpha particles.These particles collide with molecules in the air and produce other elementary particles. At sea level about one particles per square centimeter arrives every minute.The source of cosmic rays is uncertain . They possibly come from exploding stars(supernovas).
 |
| Picture of Bubble or Cloud Chamber. |
A bubble chamber photograph .A bubble chamber contains liquid hydrogen. When charged particles enter the machine , tiny bubbles form along their tracks, which can be photographed. When they collide with protons in the hydrogen nucleus other elementary particles are produced forming additional tracks .The shape of the track helps to identify the particles.
Medium and heavy particles like the mesons, the proton , and the neutron are now thought by most scientists to be made of other particles. These particles are called quarks of which there are six type of flavours: Up, Down, Strange, charmed, top and bottom.They have -1/3 or + 2/3 the charge of an electron. They combine to form protons or neutrons as in the diagram.
 |
| particle-accelarator in CERN |
Part of the proton accelerator at C E R N, Geneva .To produce protons of high energy , their initial velocity must be greatly increased .This is done electrically. The machine is ring shaped . Magnets are use to keep the protons on their circular track , in a narrow beam.
 |
| Introduction picture of Radioactivity |
RADIOACTIVITY:
An atom consists of a number of electrons moving around a central nucleus.The nucleus contains tiny particles called protons and neutrons. The nuclei of any particular element such as a carbon, always have the same number of protons, equal to the number of orbiting electrons.The positive charge of the protons is thus balanced by the negative charge of the electrons. However, the number of neutrons in the nucleus of an element can vary. Atoms of an element with the same number of protons in their nuclei but a different number of neutrons are isotopes of that element. Every element has several isotopes.
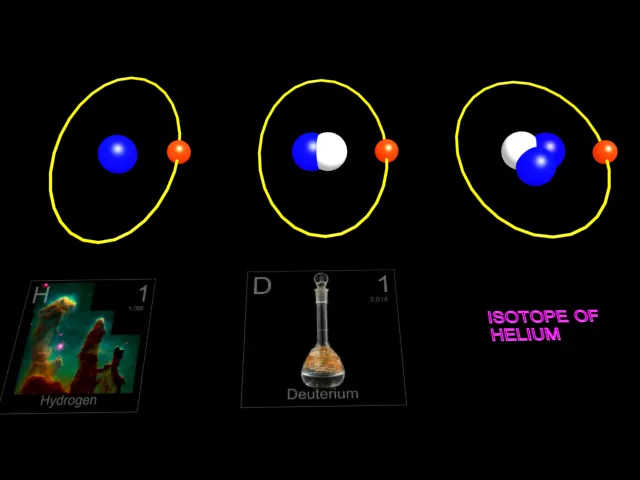 |
| The three isotopes of hydrogen. |
Animation of Isotopes of Hydrogen
The nuclei of many isotopes always remain unchanged . They are stable isotopes. Other nuclei are unstable.At moment they can emit energy, in the form of radiation, in order to reduce this instability. These are nuclei of radioactive isotopes, called radioisotopes for short . Radioactivity was first reported in 1896 by Becquerel. He found that some form of energy was being emitted by uranium salts. It was found that this emission came from the nucleus, and did not involve the orbiting electrons.
ALPHA AND BETA PARTICLES:
 |
| Energy Level of Alpha and Beta Particles |
A radioisotope can lose energy in various ways, but two important processes are the emission of an alpha particle and the emission of a beta particle. An alpha particle consists of two protons and two neutrons . It is actually the nucleus of a helium atom. After its nucleus has emitted an alpha particle, a radioisotope is changed into the isotope of another element having two less protons . The weight of the nucleus is reduced by losing the alpha particle.
A beta particle is an electron. There are no electrons in a nucleus, however, so where does it come from? It results from the sudden changes or decay of a neutron into a proton, an electron and another particle called a neutrino. This decay only occurs in nuclei of radioisotopes, never in the nuclei of stable isotopes.
After the emission of a beta particle, the radioisotope is transformed into an isotope of another element having one more proton than the original. The weights of these two isotopes are about equal, since the neutron and proton have approximately the same weight.
During the decay , radioisotopes may emit electromagnetic radiation. This can include X-rays and the more powerful gamma rays.
A radioisotope often decays into an isotope that is also radioactive. In turn, the second isotope may decay into a third radioisotope, and so on. This process will continue until a stable isotope is formed. The radioisotopes involved form a radioactive series.
The time taken for half of the nuclei of a radioisotope to disintegrate is called its half-life. The values of the half-life for different radioisotopes vary from a tiny fraction of a second to many million years. In radioactive material the number of nuclei of the radioisotope present grows less and less as more of them decay.
The activity of a radioactive material is measured by the number of disintegration in a second . It therefore decreases with time, the rate depending on the half life of the radioisotope.
 |
| The three isotopes of hydrogen. |
The three isotopes of hydrogen. The nucleus of the hydrogen atom normally contains one proton.Out of 100,000 atoms of hydrogen , 15 atoms will be the isotope deuterium .Its nucleus contains one proton and one neutron. Tritium, having two neutrons in its nucleus , is radioactive , forming a rare isotope of helium by the emission of a beta particle(electron).
 |
| Marie Curie, 1867- 1934, |
Marie Curie, 1867- 1934, a Polish physicist and chemist who worked in France, studying radioactivity. With her husband Pierre she discovered the radioactive elements radium and polonium. Her daughter Irene Joliot-Curie and her husband produced the first artificial radioisotope.
 |
| Antoine Henri Becquerel, 1852- 1908 |
Antoine Henri Becquerel, 1852- 1908 , a French physicist, who discovered the uranium salts could form an image on a photographic plate. He concluded that some form of radiation was emitted by uranium .This was the beginning of the study of radioactivity.

The energy of particles emitted by different radioisotopes can vary widely .The particles and rays emitted will also travel very different distances. The Beta (b) particles will be stopped by a thin steel of perspex, While the alpha(a) particles would not pass through a sheet of paper. Gamma (c) rays need thick steel , concrete or lead to stop them.
 |
| The radioactive series of Uranium |
The radioactive series of Uranium starts with the disintegration of a radioisotopes of Uranium Containing a total of 238 protons and neutrons (92 protons) .It ends in the production of a stable isotope of lead , Half - lives are shown for some of the disintegration.
 |
| The radioactive series of Uranium |
The Iodine , having a half life of eight days.After 16 days only the quarter of the original number of nuclei remain. This fraction is reduced to an eighth after 24 days and to a sixteenth after 32 days.
RADIOISOTOPES:
Several radioisotope occur naturally in the rocks on the earth`s surface. one out of every 100,000 atoms potassium is radioactive. Many rocks, including granite, contain potassium compounds. The radioisotope of potassium decays into a stable isotope of argon, the half-life being over 1000 million years. By measuring the amounts of these two isotopes present in a particular rock it is possible to calculate the the time at which the potassium isotope first started to decay.
This is the time at which the rock was formed. By finding the age of the earth. This method, called radioactive dating, gives the age of the earth as being 4000-5000 million years.
RADIOCARBON DATING:
Radiocarbon dating can be used to find the age of objects several thousand years old. These objects must be made from material that was once living, such as wood. All plants obtain carbon from the carbon dioxide in the atmosphere. This carbon contains a very small but constant proportion of a radioisotope with a half-life of 5730 years. The absorption of carbon from the atmosphere stops when the material dies. The radioisotope of carbon however, continues to decay. By determining the amounts of stable carbon and the radioisotope present in the object, its time of death can be found.
 |
| Instruments for Radiocarbon Dating |
Radioisotopes occurring naturally on earth all have extremely long half lives probably disappeared from the earth by decay thousands of millions of years ago.
Isotopes with short half-lives are very useful in medicine , industry and scientific research. So scientists produce their own supply . They are called artificial radioisotopes . most are formed by bombarding elements with low-energy neutrons.
Certain elements such as iodine , collect in one particular part or organ of the body. If the organ is not working properly, too much or too little collects there. A radioisotope has the same chemical properties as the stable isotopes of the same element. If a radioisotope is introduced into the human body, by injection say, it will follow the same path as a stable isotope of the same element.
 |
| Carbon-14 Cycle |
The amount of radiation emitted by the radio isotope can be measured very accurately . So. Doctors can use radioisotopes to trace the path of elements in the body . This may help them to diagnose a disease, such as cancer.
The streams of alpha or beta particles emitted by a radioisotope charge or ionize the material through which they pass X-rays and ultraviolet rays also ionize. These rays and particles add electrons to or remove them from the atoms and molecules of the material.This gives the atoms or molecules a negative or positive charge. The ionizing action of rays and particles is used in medicine to treat cancer.
Many molecules in the body have very complicated structures which can be damaged by ionizing radiation. It prevents the molecules from controlling processes going on in the cells. and the cells eventually die. Cancer cells are dangerously overactive and ionizing radiation can be use to kill them . Ionizing radiation is therefore useful. but it is a dangerous tool and great care has to be taken with it.
 |
| Radio Isotope |
Radioisotopes are very useful to both archaeologists and geologists.Here, the sodium - 24 radioisotopes is being used as a source of X-rays to radio graph one of the fallen stones at Stonehange, So the structure of the stone can be investigated.
A picture (Scan) of the amounts of a radioisotope present in different areas of the liver . The colours dark blue, green, through to red represent increasing amounts. The greatest quantity of radioisotopes should collect in the center of the liver .The dark blue area near the center of the scan indicates the presence of a cancer tumour.
 |
| A Cobalt Machine in a hospital. |
Animation on A Cobalt Machine in a hospital.
A Cobalt Machine in a hospital. Some radioisotopes including one cobalt , give off gamma rays. They are similar to X-rays but have a greatest energy .The machine produces a narrow beam of gamma rays that is used to irradiate a patient`s tumour.
 |
| A Geiger counter |
Animation on A Geiger counter Machine.It count the number of alpha or beta particles
A Geiger counter .It count the number of alpha or beta particles, it contains a gas at low pressure which is ionized by radiation entering the instrument .The ions produce a voltage in the electronic circuit connected to the counter.Each voltage pulse represents the passage of one particle.

























 Online Movies
Online Movies
No comments:
Post a Comment